Imagine a world where the fear of a lung cancer diagnosis is replaced by a beacon of hope. For decades, the battle against lung cancer, particularly non-small cell lung cancer (NSCLC), has been an uphill climb, often marked by aggressive treatments and challenging prognoses. But what if a new weapon emerged, one that could teach your own body to fight back with unprecedented precision? We are on the cusp of such a revolution. A groundbreaking mRNA lung cancer vaccine, BNT116, has just entered human trials, sparking immense optimism among researchers, clinicians, and patients worldwide. This isn’t just another incremental step; it’s a potential paradigm shift in how we approach one of the most formidable diseases known to humankind. This article will delve into the science behind this innovative vaccine, its potential impact, and what these early human trials mean for the future of cancer treatment.
Key Takeaways
- A New Era in Cancer Treatment: The BNT116 vaccine represents a significant leap forward, leveraging mRNA technology to train the immune system to target and destroy lung cancer cells.
- Targeting Non-Small Cell Lung Cancer (NSCLC): This vaccine specifically focuses on NSCLC, the most common and deadliest form of lung cancer, offering hope where traditional treatments often fall short.
- How it Works: BNT116 is designed to teach your immune system to recognize specific tumor-associated antigens (TAAs) present on cancer cells, effectively turning your body’s natural defenses into a potent anti-cancer army.
- Combination Therapy: The current Phase 2 trial is evaluating BNT116 in combination with cemiplimab, an existing immunotherapy, to assess both safety and efficacy.
- Global Collaboration: The trials are being conducted across multiple countries, highlighting the international effort and shared commitment to finding a cure for lung cancer.
- Early Stages, Immense Potential: While still in early human trials, the promise of an mRNA-based lung cancer vaccine offers a new ray of hope for millions affected by this devastating disease, potentially transforming the landscape of cancer care.
The Science Behind BNT116: Harnessing Your Body’s Defenses
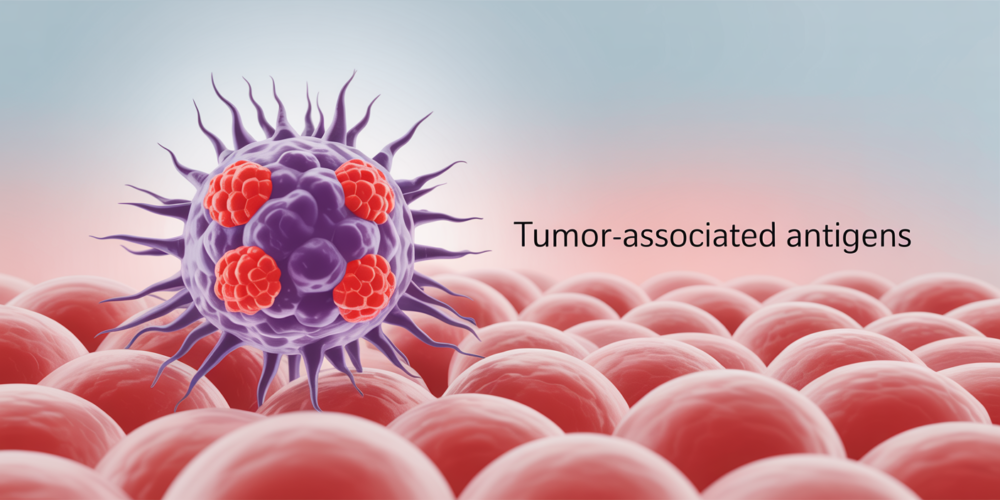
To truly appreciate the significance of BNT116, it’s essential to understand the innovative science underpinning it. For years, cancer treatment has largely relied on methods that attack cancer cells directly, such as chemotherapy, radiation, and surgery. While effective in many cases, these approaches can often come with significant side effects and may not always be able to eradicate all cancer cells, leading to recurrence. Immunotherapy, a newer frontier in cancer treatment, aims to harness the body’s own immune system to fight cancer. BNT116 takes this a step further by employing messenger RNA (mRNA) technology, a revolutionary approach that gained prominence with the development of highly effective COVID-19 vaccines.
At its core, BNT116 is designed to act as a sophisticated training manual for your immune system. Cancer cells, unlike healthy cells, often display unique markers on their surface called tumor-associated antigens (TAAs). These TAAs are like distinctive flags that signal to the immune system that something is amiss. However, cancer cells are often adept at evading immune detection, either by not presenting enough of these flags or by actively suppressing immune responses. This is where BNT116 comes in.
The vaccine contains carefully engineered mRNA sequences that carry the genetic instructions for producing several of these specific TAAs. When BNT116 is administered, your body’s cells temporarily take up this mRNA. Following these instructions, your cells then produce the TAAs, much like they would produce any other protein. Crucially, these TAAs are not part of a live virus or bacteria; they are simply protein fragments that mimic those found on lung cancer cells. Once these TAAs are produced, your immune system’s specialized cells, particularly T-cells and B-cells, recognize them as foreign. This recognition triggers a powerful immune response, teaching your body to identify and remember these specific cancer markers.
Think of it like this: your immune system is a highly trained army, but it needs to know who the enemy is. BNT116 provides the detailed intelligence, showing your immune cells exactly what lung cancer cells look like. This ‘training’ enables your immune system to mount a targeted and robust attack against existing lung cancer cells and, potentially, to prevent future cancer growth or recurrence. The beauty of mRNA technology lies in its precision and adaptability. It can be rapidly designed to target multiple TAAs, making it a versatile platform for addressing the complex and diverse nature of cancer. Furthermore, mRNA vaccines do not alter your DNA; the mRNA instructions are temporary and are naturally degraded by the body after they have served their purpose, leaving no lasting genetic footprint.
The Clinical Trials: A Glimpse into the Future

The journey from scientific discovery to a viable medical treatment is long and rigorous, marked by multiple phases of clinical trials. BNT116 has now entered human trials, a critical juncture that will determine its safety and efficacy in patients. The current Phase 2 study, known as EMPOWERVAX Lung 1 (NCT05557591), is a pivotal step in this process. This trial is designed to evaluate BNT116 not just as a standalone treatment, but in combination with cemiplimab, an anti-PD-1 antibody that is already an established immunotherapy for various cancers, including NSCLC.
Combining BNT116 with cemiplimab is a strategic approach. Cemiplimab works by blocking the PD-1 pathway, a mechanism that cancer cells often exploit to evade immune detection. By combining it with BNT116, researchers aim to create a synergistic effect: BNT116 primes the immune system to recognize cancer cells, while cemiplimab removes the brakes that cancer cells put on the immune response. This dual approach has the potential to unleash a more powerful and sustained anti-tumor attack.
The EMPOWERVAX Lung 1 trial is specifically enrolling patients with advanced non-small cell lung cancer whose tumors express PD-L1 ≥50%. This patient population is particularly challenging to treat, making any positive outcomes in this trial especially significant. The primary objectives of the study are to assess the safety and tolerability of the BNT116-cemiplimab combination, and to compare its effectiveness against cemiplimab monotherapy. Researchers will be meticulously monitoring for any side effects, evaluating how much of the study drug is present in the blood over time, and checking for the development of antibodies against the study drugs, which could impact their effectiveness.
This global endeavor involves 34 medical centers across seven countries, including major sites in the UK, US, and Germany. This international collaboration underscores the urgency and shared commitment of the scientific community to address the unmet needs of lung cancer patients. While the study is estimated to complete its primary phase by March 2027 and the full study by June 2027, the initiation of these human trials represents a monumental achievement. It signifies that the vaccine has successfully passed preclinical testing and has demonstrated enough promise to warrant investigation in human subjects. The data gathered from these trials will be crucial in determining the future trajectory of BNT116 and its potential to revolutionize lung cancer treatment.
Broader Implications and Future Outlook: A New Dawn for Cancer Care
The implications of a successful mRNA lung cancer vaccine extend far beyond just non-small cell lung cancer. The very platform of mRNA technology holds immense promise for developing vaccines against a wide array of cancers, potentially revolutionizing oncology as we know it. If BNT116 proves safe and effective, it could pave the way for similar mRNA-based therapies targeting other solid tumors and even blood cancers. The ability to rapidly design and produce mRNA vaccines, as demonstrated during the COVID-19 pandemic, means that future cancer vaccines could be developed and deployed with unprecedented speed, adapting to the evolving nature of cancer.
References
- ClinicalTrials.gov. (n.d.). A Trial to Learn How the Cancer Vaccine BNT116 in Combination With Cemiplimab Works and How Safe the Combination is in Adults With Advanced Non-small Cell Lung Cancer (EMPOWERVAX Lung 1). Retrieved from https://clinicaltrials.gov/study/NCT05557591
- BioNTech. (n.d.). Clinical Trial Evaluating the Safety, Tolerability and Preliminary Efficacy of BNT116 in Patients with Advanced Non-Small Cell Lung Cancer. Retrieved from https://clinicaltrials.biontech.com/trials/BNT116-01
- Clinical Trials Arena. (2024, August 26). BioNTech initiates global trials of mRNA-based lung cancer vaccine. Retrieved from https://www.clinicaltrialsarena.com/news/biontech-trials-lung-cancer-vaccine/
- The Guardian. (2024, August 23). World-first lung cancer vaccine trials launched across seven countries. Retrieved from https://www.theguardian.com/society/article/2024/aug/23/world-first-lung-cancer-vaccine-trials-launched-across-seven-countries