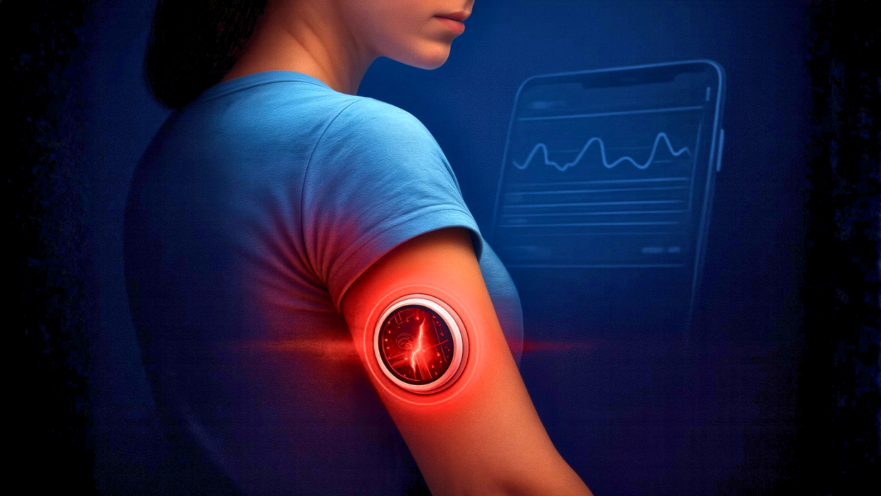
Abbott, one of the world’s biggest health care companies, has recalled millions of blood glucose sensors across more than a dozen countries, sparking alarm for people with diabetes who rely on these devices to stay safe. At least seven deaths and hundreds of serious injuries have now been linked to defective FreeStyle Libre 3 and Libre 3 Plus sensors.
Key Takeaways
- Abbott has recalled certain FreeStyle Libre 3 and Libre 3 Plus blood glucose sensors after reports of inaccurate readings.
- Faulty sensors have been linked to seven deaths and 736 serious health incidents, including 57 in the U.S.
- Inaccurate readings could lead to dangerous health decisions for people with diabetes.
- Millions of affected devices were distributed globally, including in the U.S., Europe, Canada, and New Zealand.
- Abbott says it has identified the issue and is replacing the faulty sensors without expecting major supply disruptions.
Why Was the Recall Issued?
This massive recall came after Abbott discovered that sensors made on a specific production line were providing inaccurate blood glucose readings. These errors increase the risk of life-threatening situations for people with both type 1 and type 2 diabetes. Accurate blood sugar monitoring is literally a matter of life and death for these patients.
What Are the Dangers of Inaccurate Blood Sugar Readings?
False readings can trigger poor decisions. A falsely high reading could cause someone to miss a low blood sugar episode, a potentially deadly situation known as hypoglycemia. On the flipside, a falsely low reading might lead someone to overdose on insulin, also causing dangerously low blood sugar. Both situations can end in seizures, unconsciousness, coma, or even death if not caught in time.
How Many Devices Are Affected?
Abbott says around three million defective glucose sensors were distributed in the U.S. alone, with millions more sold in Austria, Belgium, Canada, Denmark, Finland, France, Germany, Italy, Luxembourg, the Netherlands, New Zealand, Norway, Spain, Sweden, Switzerland, and the U.K. The recall covers FreeStyle Libre 3 and Libre 3 Plus sensors with certain model numbers.
How Do These Sensors Work?
The sensors use a tiny, flexible filament inserted just under the skin, measuring glucose in the fluid between cells. An enzyme reacts with glucose to create a small electric current, which the device translates into a glucose reading on your phone. With faulty sensors, that reading simply can’t be trusted.
What Should You Do If You Have a Recalled Sensor?
Abbott and the FDA warn that anyone using an affected device should stop immediately. Contact Abbott or your health care provider for replacement guidance. The company says it’s already resolved the problem in its production line and continues to ship safe products.
What Are the Long-Term Risks?
Using faulty glucose sensors can have hidden long-term effects—uncontrolled blood sugar spikes or drops can result in nerve damage, kidney disease, heart problems, and increased risk for emergencies like coma. That’s why catching this recall and acting quickly matters so much for your health.
Conclusion
If you or someone you care about uses Abbott’s FreeStyle Libre 3 or Libre 3 Plus, double-check the model numbers and contact your health care provider right away. Never ignore a recall—when it comes to diabetes and blood sugar control, accuracy saves lives.
Scientific References
- Abbott Medical Device Recalls
- FDA Safety Communication on Abbott FreeStyle Libre Recall
- CDC Diabetes Statistics
Stay alert for updates from manufacturers and always prioritize your or your loved ones’ safety by responding quickly to health device recalls.